The original presentation is available:
https://drive.google.com/file/d/1wKgp8XgoUP1alau_f1YxlaFBGrcVp_hw/view?usp=drivesdk
Why are Humans Aging? Part 3.
Mitochondrial Dysfunction
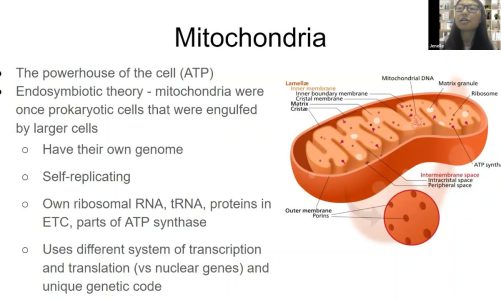
Mitochondria are intracellular organelles that provide energy to the cell. Hundreds of mitochondria, which are highly active components, are found in each cell. They replicate and fuse with other mitochondria, exchange molecular machinery, and can even transfer between cells. The majority of their DNA has migrated into the cell nucleus, but a few genes remain to form circular mitochondrial DNA. Age-related mitochondrial dysfunction and an increase in reactive oxygen species (ROS) production can cause cellular damage and accelerate aging.
ROS are chemically active oxygen-containing substances produced in excess by dysfunctional mitochondria in comparison to the body’s ability to neutralize them. This is referred to as oxidative stress. Peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha oxygen are all examples of ROS. ROS in excess damage polyunsaturated fatty acids (PUFA), DNA, RNA, and proteins, all of which contribute to aging. Mitochondrial DNA is especially vulnerable to oxidation, which is a major cause of aging. Antioxidants, particularly glutathione, are important in lowering ROS levels. Glutathione production, on the other hand, declines with age, resulting in accelerated aging.
It is known that mitochondria convert saturated fatty acids to ATP more easily than glucose and unsaturated fatty acids. Oxidation of glucose in mitochondria occurs faster, but with an excessive release of the so-called Reactive Oxygen Species (ROS) which are chemically active substances containing oxygen. Therefore, avoiding carbohydrates and following a Ketogenic/Carnivore diet with animal (saturated) fats is the best choice to slow down aging. Intermittent fasting and training during such fasting would allow us to cycle a process of mitophagy to remove defective and old mitochondria and replace them with new ones.
Mitochondria can move, split in half, and fuse with other mitochondria. This fusion and fission cycle allows defective mitochondrial DNA copies to be eliminated. Intense aerobic exercise increases the number of mitochondria in cells. Saunas and exposure to colder temperatures also aid in the formation of new mitochondria. Increasing the number of mitochondria reduces stress on each mitochondrion and reduces ROS levels in the cells. However, excessive anaerobic training intensity can result in the accumulation of ROS and damage of cell membranes and even to apoptosis.
In the elderly, mitochondrial dysfunction develops, particularly in muscle cells, resulting in increased ROS production and an inability to convert fatty acids to energy. Instead, glucose is used as an energy source, and ATP molecules are primarily produced during the glycolysis process in cytoplasm of cells. Meanwhile, liquid fatty acid drops accumulate in cells. Deficit in energy production is causing the development of sarcopenia. Intense physical activity, intermittent fasting, and low-carbohydrate diets, on the other hand, may help improve mitochondrial function.
In a mouse study, researchers activated a mutation that resulted in a loss of mitochondrial function, similar to what happens in aging humans. Within four weeks, the mice developed gray hair, hair loss, and lethargy. The mice’s skin started to sag after four to eight weeks, resembling both intrinsic and extrinsic aging, such as that caused by smoking or sun exposure. The mice’s skin’s outer layer thickened, the number of skin cells increased, there was inflammation, and the hair follicles were dysfunctional, all of which are symptoms of extrinsic aging in humans. The researchers, however, were able to halt the mutation and reverse the effects.
source