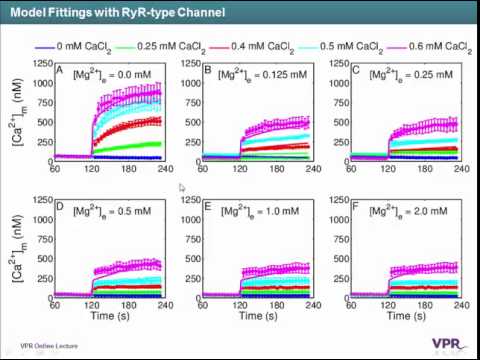
Cardiac mitochondria can act as a significant Ca2+ sink and shape cytosolic Ca2+ signals affecting various cellular processes, such as energy metabolism and excitation-contraction coupling. However, different mitochondrial Ca2+ uptake mechanisms are still not well understood. In this study, we analyzed recently published Ca2+ uptake experiments performed on isolated guinea pig cardiac mitochondria using a computer model of mitochondrial bioenergetics and cation handling. The model analyses of the data suggest that the majority of mitochondrial Ca2+ uptake, at physiological levels of cytosolic Ca2+ and Mg2+, occurs through a fast Ca2+ uptake pathway, which is neither the Ca2+ uniporter nor the rapid-mode of Ca2+ uptake. This fast Ca2+ uptake component was explained by including a biophysical model of ryanodine receptor (RyR) in the computer model. However, the Mg2+-dependent enhancement of the RyR adaptation was not evident in this RyR-type channel, in contrast to that of cardiac sarcoplasmic reticulum RyR. The extended computer model is corroborated by simulating an independent experimental dataset, featuring mitochondrial Ca2+ uptake, egress and sequestration. The model analyses of the two datasets validate the existence of two classes of Ca2+ buffers that comprise the mitochondrial Ca2+ sequestration system. The modeling study further indicates that the Ca2+ buffers respond differentially depending upon the source of Ca2+ uptake. In particular, it suggests that the Class 1 Ca2+ buffering capacity is auto-regulated by the rate at which Ca2+ is taken up by the mitochondria.
source
Ca2+ dynamics in isolated cardiac mitochondria predicts two distinct modes of Ca2+ uptake